Minakem Louvain-la-Neuve has been manufacturing Active Pharmaceutical Ingredients (APIs) for over 20 years.
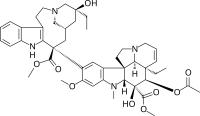
The site has a longstanding expertise in purification and the production of vinca derivatives (complex flower extracts derived from natural feedstock) and their transformation into APIs.
Additionally, the site has experience in lyophilizing APIs for injectable applications, particularly in oncology treatments.
The site also manufactures controlled substances, including Remifentanil API.
Strategically located in the heart of Europe, near Brussels, the site exports 100% of its production globally.