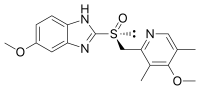
Minakem Dunkerque has been manufacturing Active Pharmaceutical Ingredients (APIs) for over 30 years. The site, formerly AstraZeneca’s legacy API facility, is dedicated to the production of esomeprazole, omeprazole, and budesonide APIs.
The site has extensive expertise in manufacturing large quantities of APIs and micronizing them. It also manufactures corticosteroids, including Budesonide API.
Strategically located in the heart of Europe, near the Dunkerque and Antwerpen harbors, the site exports more than 50% of its production globally.