 Abstract:
Abstract:
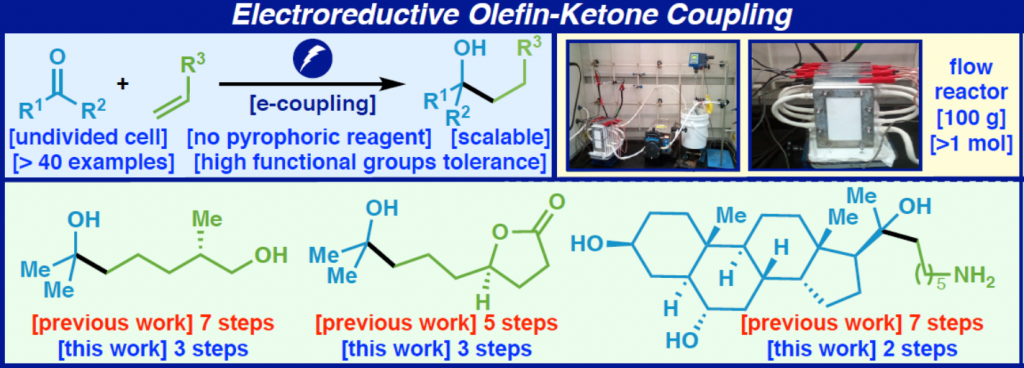
A user-friendly approach is presented to sidestep the venerable Grignard addition to unactivated ketones to access tertiary alcohols by reversing the polarity of the disconnection. In this work a ketone instead acts as a nucleophile when adding to simple unactivated olefins to accomplish the same overall transformation. The scope of this coupling is broad as enabled using an electrochemical approach, and the reaction is scalable, chemoselective, and requires no precaution to exclude air or water. Multiple applications demonstrate the simplifying nature of the reaction on multistep synthesis, and mechanistic studies point to an intuitive mechanism reminiscent of other chemical reductants such as SmI2 (which cannot accomplish the same reaction).
Access the full article now
Source
Publication Date: December 1, 2020
Graphical Abstract
Authors:
Pengfei Hu, Byron K. Peters, Christian A. Malapit, Julien C. Vantourout, Pan Wang, Jinjun Li, Lucas Mele, Pierre-Georges Echeverria, Shelley D. Minteer*, and Phil S. Baran*
You may also ask to get in contact with our technical team through our contact form
Minakem Recherche, 145 Chemin des Lilas, 59310 Beuvry la Forêt, France
