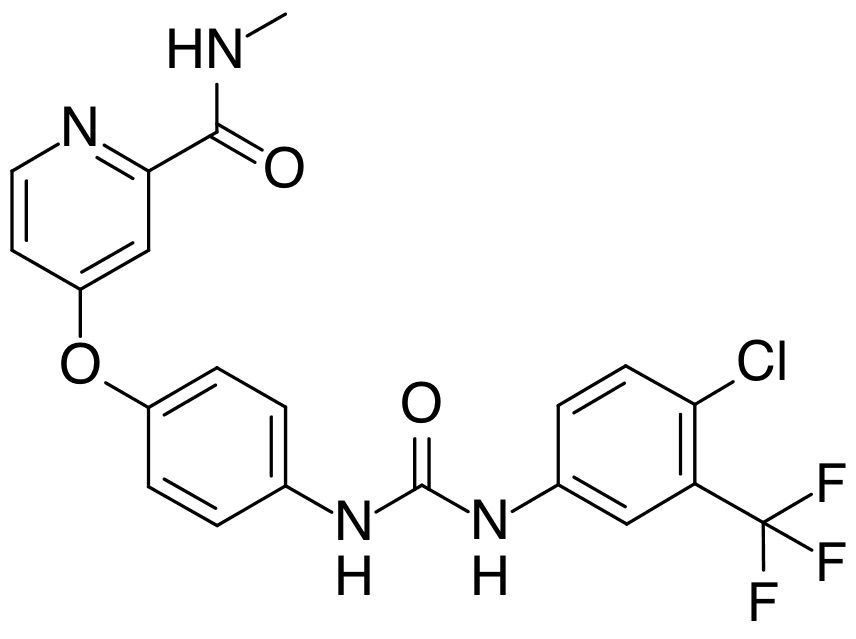
sorafenib tosylate API has been developed in Minakem’s Canadian facility (Delmar Chemicals Inc.) in 2016.
From a regulatory perspective, Minakem offers:
– An American DMF (Drug Master File) #32140
Minakem’s sorafenib brings:
– Form 1
About Minakem Montreal’s Expertise
Minakem Montreal (Delmar chemicals Inc.) has been manufacturing Active Pharmaceutical Ingredients (APIs) since 1941. It is the largest API facility in Canada, benefiting from a strong scientific and technological environment. Located close to its U.S. customers in the New Jersey and Boston areas, Montreal also has a strong tradition of serving the entire North American market, as well as Latin America, Europe, Japan, China, and India.
About Minakem’s Network
Minakem Montreal works daily with Minakem’s other European facilities, leveraging their complementary expertise, such as solid engineering, analytics, global regulatory affairs, and capabilities to optimize competitiveness.
Minakem has over 40 years of expertise in developing, scaling up, registering DMFs, and manufacturing complex APIs, including chiral small molecules, steroids, and highly potent APIs (HPAPIs, primarily used in oncology treatments).
Strong supply chain performance is becoming critical to our customers’ success.
Minakem’s operations focus on producing safe products for patients, achieving high OTIF performance, providing strong quality support, and consistently seeking solutions to meet customers’ unexpected market demands.
More data on sorafenib API
sorafenib’s data are avilable on Pharmaoffer.
sorafenib additional data are available on Wikipedia
-
Category
-
CAS NO.
2056030-06-7
-
Regulatory Documentation
USDMF
-
Certificates
GMP
USFDA audit
-
Structure