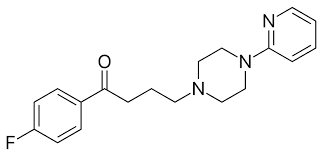
The Azaperone API was developed at Minakem’s Leuna facility in 2009 and has since been transferred to Minakem’s French facility in Beuvry-la-Forêt.
The Azaperone API CAS number is 1649-18-9
From a regulatory perspective, Minakem offers:
– a European Drug Master File (DMF) CEP since 2013
Therapeutical Class and Mechanism of Action
Azaperone API belongs to the butyrophenone class of antipsychotics and is primarily used as a sedative and tranquilizer in veterinary medicine. It is commonly used to manage stress and aggression in pigs and other big animals during handling, transport, or medical procedures.
Azaperone API works by blocking dopamine receptors in the central nervous system (CNS), leading to the following effects:
– Sedation and Anxiolysis Effect to reduce excitability, leading to sedation and a calming effect.
– Antipsychotic Effect to reduce aggression and agitation behaviors.
– Muscle Relaxation Effect to aid in the reduction of stress-induced behaviors.
Azaperone API has minimal effects on respiratory and cardiovascular systems at therapeutic doses, making it relatively safe for use in veterinary contexts.
About Minakem Beuvry-la-Forêt’s Expertise
Minakem Beuvry-la-Forêt was the first site acquired by Minafin (formerly SEAC) in 2005. The site has extensive expertise in R&D and analytical development and has been manufacturing Active Pharmaceutical Ingredients (APIs) for over 20 years.
In 2023, a significant investment of 35 million EUR was made to enhance its competitiveness in API manufacturing and to improve its HSE ranking through the implementation of a modern COV (Volatile Organic Components) reduction system.
The site specializes in low-temperature chemistry (e.g., Suzuki coupling, lithiation), cyanuration, bromination, hydrogenation, and more. Additionally, it excels in solid engineering design, enabling the efficient management of polymorphism and particle size distribution (PSD).
Strategically located in the heart of Europe, between Lille, Paris, and Brussels, the site exports 90% of its production globally.
About Minakem’s Network
Minakem Beuvry-la-Forêt collaborates daily with Minakem’s other European facilities and with Minakem Montreal, leveraging their complementary expertise in solid engineering, analytics, global regulatory affairs, and other capabilities to enhance competitiveness.
Minakem brings over 40 years of experience in developing, scaling up, registering Drug Master Files (DMFs), and manufacturing complex Active Pharmaceutical Ingredients (APIs), including chiral small molecules, steroids, and highly potent APIs (HPAPIs)—primarily used in oncology treatments.
As supply chain performance becomes increasingly critical to customers’ success, Minakem focuses on delivering:
- Safe products for patients,
- High On-Time-In-Full (OTIF) performance,
- Robust quality support, and
- Innovative solutions to address customers’ unexpected market demands.
More data on Azaperone API
Azaperone’s data are available on Pharmaoffer.
Azaperone additional data are available on Wikipedia
-
Category
-
CAS NO.
1649-18-9
-
Regulatory Documentation
CEP
-
Certificates
GMP
EDQM audit
Nitrosamines
TSE/BSE
Non GMO
Hallal
-
Structure